Tags
animals, biology, Developmental biology, Education, evolution, Genetics, imprinting, nursing, offspring, Popular science, science
 This is a story about a gene which makes nursing mice produce more nutritious milk while also making their offspring less demanding. The gene serves to balance nutrient supply and demand between the mother and pup. If the gene is knocked out, the mother’s milk is less rich, but the pups are more demanding, evening out the impact. Things only go wrong when there’s a mismatch. If pups with a defective copy of the gene feed from a normal mother, their increased demand makes them grow larger than normal. Conversely, pups with a good copy end up smaller if they feed from a mother lacking a working copy, since her milk is less nutritious.
This is a story about a gene which makes nursing mice produce more nutritious milk while also making their offspring less demanding. The gene serves to balance nutrient supply and demand between the mother and pup. If the gene is knocked out, the mother’s milk is less rich, but the pups are more demanding, evening out the impact. Things only go wrong when there’s a mismatch. If pups with a defective copy of the gene feed from a normal mother, their increased demand makes them grow larger than normal. Conversely, pups with a good copy end up smaller if they feed from a mother lacking a working copy, since her milk is less nutritious.
So far we’ve talked about mothers and pups, but there’s a third player in this dance: the pup’s father. We inherit two copies of every gene, one from each of our parents, so the father has a say in which version of the gene the pup gets. For a gene like this, it’s in the father’s best interest to pass on a defective copy in an effort to knock out the gene in their offspring. Without a working copy, the pups will either be normal sized (if the mother also has a defective copy) or oversized (if she doesn’t) — either way, they end up better off than any siblings with a working copy. It seems like a win-win situation for the fathers; of course, things aren’t actually that easy.
The father’s winning scenarios come at the expense of the mother and her other pups, and she’s at the focus of this particular evolutionary conflict. She’s the one that has to provide nutrients, and she wants to make sure that all her pups do well — not just the demanding pups with (genetically) manipulative fathers. The mother has a lot more at stake, so her priorities have shaped the evolution of this gene and its role in development. Both copies (maternal and paternal) of most of our genes are usually expressed, but this gene is one of the exceptions. In a process called ‘imprinting’, only one of the copies is active; the other copy gets switched off. The father’s version is active in the brain and spinal cord, but the mother’s version calls the shots everywhere else. This means that the same version of the gene — the mother’s copy — is controlling both the pup’s nutrient demand and the mother’s mammary glands, coordinating the interaction to ensure a comfortable balance. The father doesn’t get a chance to shift things his way, since his version of the gene simply isn’t in play.
The story doesn’t end there, though. The gene doesn’t just control the balance of supply and demand between the mother and pup; it also controls the amount of lean tissue and fat in the pup. Pups with defective versions grow into adults with more lean mass and less fat. By fostering pups with a defective copy with mothers with a normal copy (and vice versa), researchers made a startling discovery. The lean/fat ratio in the pups doesn’t depend solely on what version of the gene the pup has, but also on the copy in the nursing mother. The pup’s copy of the gene determines how much lean tissue it has; knocking out the gene leads to more lean mass. The amount of fatty tissue, on the other hand, is decided by which version the pup’s nurse has. Pups nursed by mothers with a defective copy have less fatty tissue but the same amount of lean tissue.
So it turns out this gene is crucial to the pups’ development in two different ways. The versions in the mother and pup cooperate to coordinate the pups size; as long as the two are the same, everything is fine, but a mismatch leads to trouble. The mother’s copy of the gene is active in the pup in most of the pup — the father’s copy gets switched off — so there usually isn’t a mismatch (unless nosy researchers start swapping pups around in fostering experiments). At the same time, the gene in the mother is controlling the amount of fatty tissue in her pup, while the pup’s copy controls its lean mass. If the mother and pup both have defective copies, the pup will be normal sized but end up with more fat and less lean tissue. For those of you who are more visual, here’s a wonderful summary picture from the research paper:
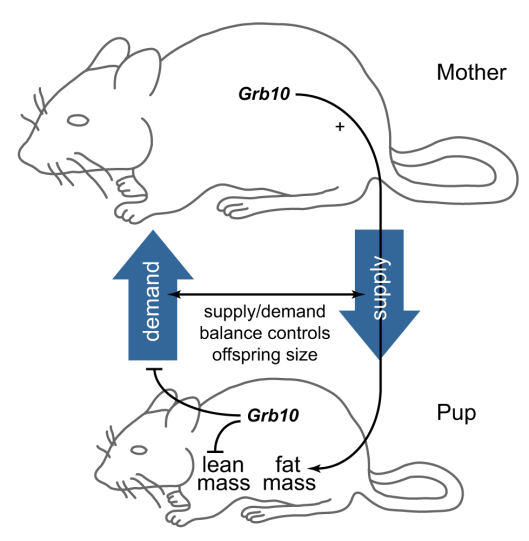
The gene (Grb10) has controls supply & demand in the mother & pup, as well as the lean/fat ratio in the pup. (Image credit: Figure 5 from the paper)
Of course, this research was all conducted in mice, and it’s not clear how well the results will apply to humans, since we have a pretty strange offspring-mother relationship where the fetus voraciously tries to dominate the mother’s tissues and hijack control of its nutrient supply. Nevertheless, it’s a pretty amazing system, the result of an interplay between the conflicting interests of several actors and the evolutionary and developmental forces that have reconciled them.
Ref
Cowley M, Garfield AS, Madon-Simon M, Charalambous M, Clarkson RW, Smalley MJ, Kendrick H, Isles AR, Parry AJ, Carney S, Oakey RJ, Heisler LK, Moorwood K, Wolf JB, & Ward A (2014). Developmental programming mediated by complementary roles of imprinted Grb10 in mother and pup. PLoS biology, 12 (2) PMID: 24586114

Yikes, that’s confusing. I’m not sure I understand the father’s genes’ roles in all this…
In this context, the father’s genes don’t get to play a role. The pups inherit one copy of the gene from him, but it’s switched off everywhere (except the central nervous system), leaving only the copy they inherited from their mother active. That enables the mother & pup to coordinate the supply & demand of nutrients without letting the father manipulate the interaction (via the copy the pup inherited from him).
I hope that helps clarify things! If not, let me know and I’ll come up with a better way to explain it. 🙂
Why is the gene turned off everywhere else except the central nervous system? Is that natural or something the experimenters did? For either case, why? (The first case would be much more intriguing. What’s the benefit to an organism in passing on a gene just so that it will be turned off?)
Thanks for the questions!
The father’s copy of the gene is switched off by a process called imprinting. It wasn’t something the experimenters did. For most of our genes, both copies (one from each parent) are active, but for a small percentage of genes only one copy is used in each cell/tissue.
Even though both parents invest in the offspring, they can have conflicting interests. For example, if the male is unlikely to mate with the same female again, it’s best for him if his offspring take as much food/nutrients from the mother as possible. The mother, on the other hand, wants to preserve her energy for other (possibly future) children, so she tries to limit what she gives to each offspring. Genetic imprinting is one way these evolutionary conflicts get resolved — one parent’s copy of a gene deactivate the other’s to protect their interests.
Different copies can be active in different tissues; in this case, the father’s copy is active in the brain and spinal cord and the mother’s copy is used elsewhere. In the central nervous system, the father’s copy seems to somehow regulate social behaviour — mice which get a defective copy from their father show “increased social dominance”. So the copy from the mother acts in the body to regulate nutrient supply/demand, while the copy from the father acts in the nervous system to control social behaviour. There’s one more wrinkle: the effect of the mother’s copy also depends on what version of the gene the nursing mother has. The pup & nursing mother should have matching versions for the pup to develop normally. (That’s what’s shown in the image at the end of the article.)
I hope that helps! Feel free to ask if something is still unclear.
Ah, that does make it clearer. Very interesting that one set of genes can deactivate another set of genes. Jeez, that must get confusing. No wonder we’re all just a bit whacked. 😉
So, I wonder…are there ways of suppressing the imprinting (correct me if I’m using that term incorrectly)? Meaning, say I wanted my father’s genes and not my mother’s, could I (or researchers) change the apparent override?
I don’t know of any techniques for doing that, and I couldn’t find anything when I looked, but it’s possible I’m missing something. In principle, it’s certainly possible — just quite challenging. Of course, to have the full impact, it would have to have been done before birth (or even conception), since some imprinted genes (including this one) seem to play a role in development and embryogenesis.
Wow – very interesting. Thanks for answering my questions. 🙂
You’re quite welcome!
An unrelated finding (yet relevant to your post title) shows that breast milk contains microRNAs that are pretty resistant to harsh conditions, suggesting that they might contribute to the development of an infant’s immune system. Very interesting!
http://www.ijbs.com/v08p0118.htm
Wow, that’s really interesting! I’ll have to look into how much more we know about that and keep an eye out for follow-up studies. Thanks for sharing it!